What are the steps to becoming GMP certified?
What are the steps to becoming GMP certified?
Achieving GMP certification is usually a large project that takes several months to complete. The major steps to becoming GMP certified are listed and discussed below.
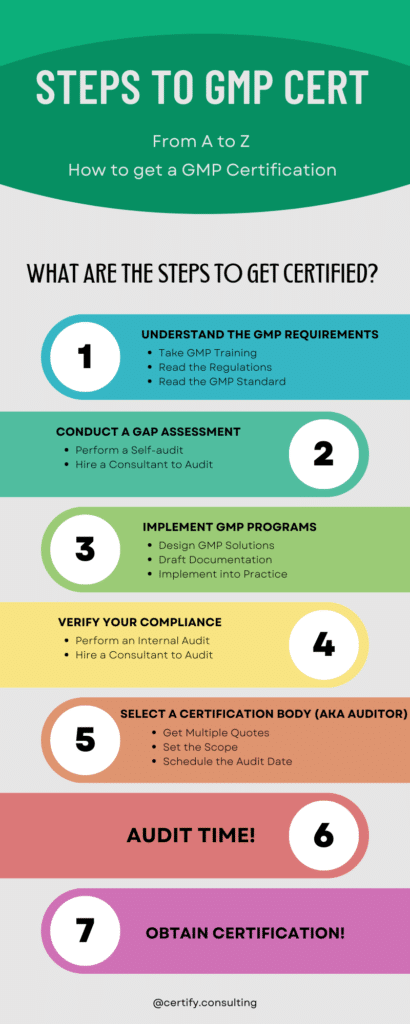
1. Understand the GMP Requirements
The best way to understand the GMP requirements is to obtain GMP training from an accredited institution. GMP training is required anyway so start off on the right foot by getting it right away. GMP is complicated enough without the additional context that proper GMP training will provide. We provide a free written training as part of this blog. Go to our GMP Training category.
2. Conduct a Gap Assessment
A gap assessment is difficult to do, even with institutional GMP training. Experience with certification audits audits is the best preparation to perform an accurate gap assessment. A GMP consultant can help greatly in this step of the process.
3. Implement the GMP Programs
Once you know your gaps, then you need to design a solution to those gaps that aligns with current best practices and the vision and values of your organization. Once you know what those solutions look like, you can draft the necessary GMP documentation and implement them in practice. If you don’t like drafting auditable procedures, then a GMP consultant can be a great help in this step as well.
4. Verify Your Compliance
Once your implementation is complete, then you should verify that all has been implemented as planned. GMP requires that you do a regular “Internal Audit” in any case, so use your first internal audit to verify that everything is in order before scheduling the certification audit. If you do not feel that you are objective enough to fairly self-audit, then a GMP consultant can help with this step as well by performing your first internal audit for you. This is also a good opportunity for the consultant to train you how to properly perform internal audits.
5. Select a Certification Body
A certification body is the organization that sends the auditor and certifies you. Not all certification bodies are the same. They have slightly different GMP standards and certification processes, as well as different costs and fees. Also, the quality of the auditor that you receive can depend on which certification body you choose. Be sure to get multiple quotes, and to ask about their processes. You should discuss scope the scope of the audit with the auditor before the audit to ensure you are all on the same page. A GMP consultant can also help you with selecting a certification body that aligns with your needs and budget.
6. AUDIT TIME!
An audit is always a little stressful, but if you’ve done your homework and relied on experienced advisors and consultants to verify your readiness, then you have nothing to worry about!
7. Obtain Certification
When you pass your audit, it will take some time for the certification body to provide you with the certificate. Almost always the auditor will find some nonconformities. This is common and usually does not result in a failed audit, but you will be expected to quickly correct those minor issues before the certificate is issued.
