What are the GMP requirements? List them all.
What are the GMP requirements? List them all.
The best place to go for a list of all of the GMP requirements is to the source of the requirements. The source of the requirements is the federal code for each of the FDA-regulated GMP industries. For you deep divers, the code is linked below for your reference.

21 CFR Part 700s (but I recommend referring instead to ISO 22716)
21 CFR Part 111 – Dietary Supplements
21 CFR Part 211 – OTC Drugs and Pharmaceuticals
Another way to learn the GMP requirements is to visit our GMP Training posts where each GMP subject is addressed individually in a written training format.
The below diagram shows the general GMP subjects and which apply to each industry.
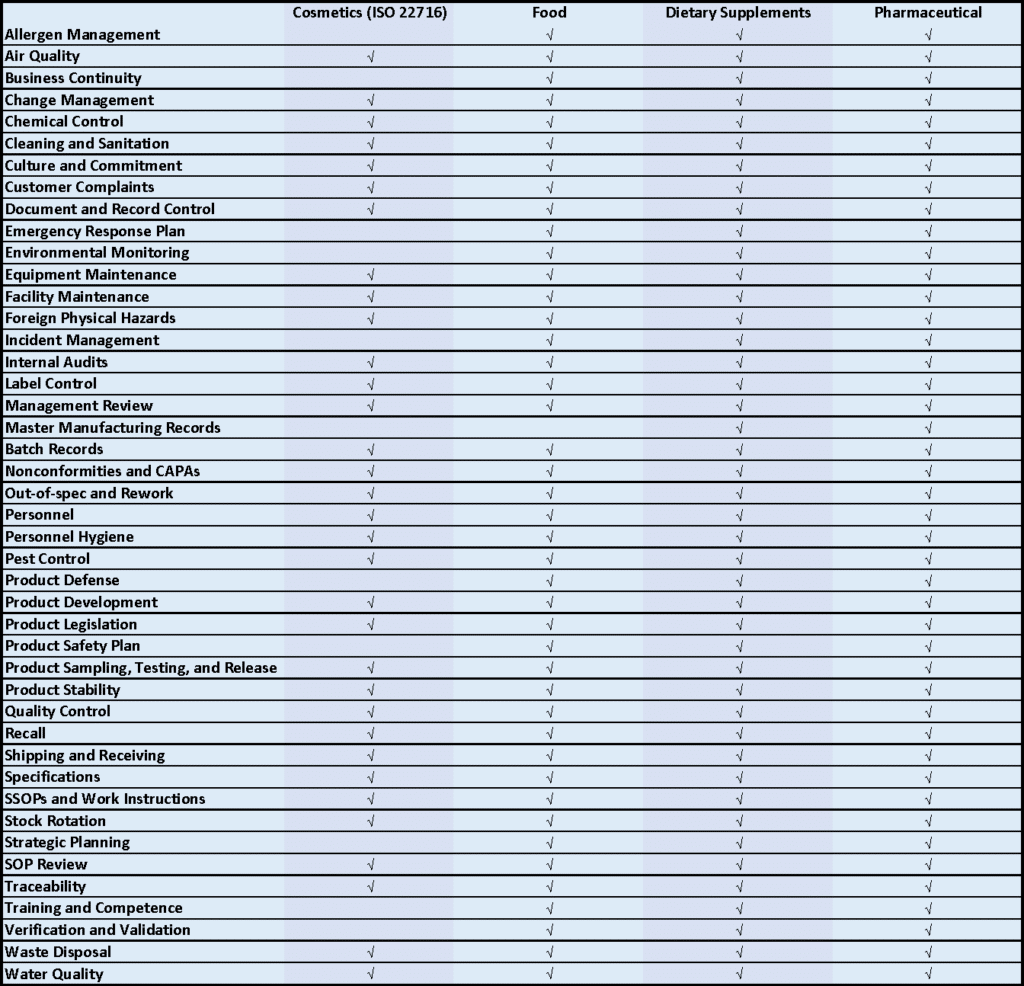
The GMP requirements do not divide perfectly into these GMP subjects, and the accuracy of the table is therefore somewhat debatable. The best approach is to build your implementation checklist off of the regulations themselves.
